Balancing Net Ionic Redox Equations
Caracterdesign Getty Images Great job. - -- --- ---- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- ----- -----.
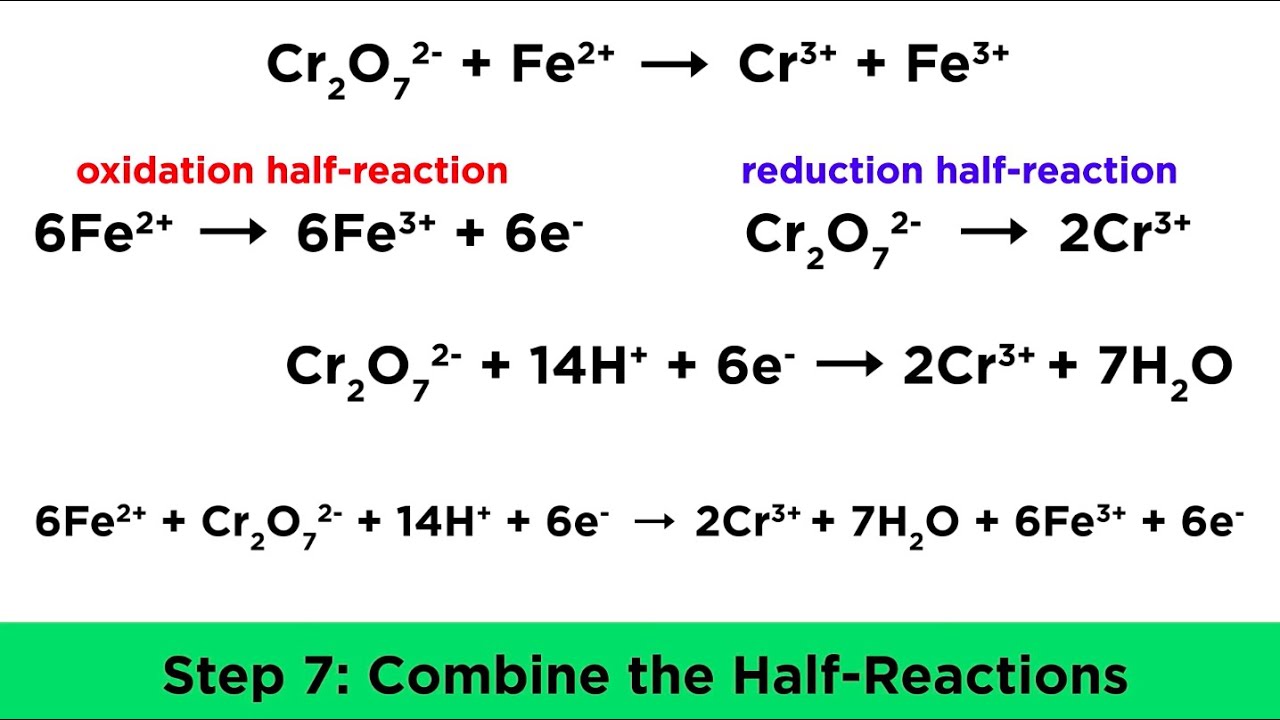
Balancing Redox Reactions In Acidic And Basic Conditions Youtube
Redox reactions Opens a modal.

. Equation balancer is an online tool for balancing chemical equations. You did so well on this quiz you could tutor others on how to balance equations. In reactions involving many compounds equations may then be balanced using an algebraic method based on solving set of linear equations.
Formatting them differently bold would help student identify during the learning phase. This course continues the examination of principles and applications of chemistry that was begun in CHM150. The same goes for example balancing redox equations in.
Ionic chemical equations are slightly different from that of a classic case of chemical equations. Coefficients represent both the basic unit and mole ratios in balanced equations. The concept of complete and net ionic equations should have been integrated within the precipitation and acidbase reactions instead of standing alone in the balancing equations section.
Assign variables to each coefficient. Redox flow batteries are a critical technology for large-scale energy storage offering the promising characteristics of high scalability design flexibility and decoupled energy and power. While fully ionic bonds are not found in nature many bonds exhibit strong.
Topics include properties of solutions acids and bases kinetics equilibrium thermodynamics oxidation reduction ionic and redox equations and electrochemistry. Some examples of cations are Calcium Ca 2 Potassium K hydrogen H. Balancing Equations Test Questions.
Predicting Precipitates and Net Ionic Equations Precipitation Reactions. Predicting Precipitates and Net Ionic Equations Video Take Quiz. Predicting Precipitates and Net Ionic Equations Video Take Quiz.
How to Balance Net Ionic Equations. These electrolytes are split up and written as individual ions. Modification of work.
Balanced Equation Example Problem. May be interpreted as representing the difference between the energy produced by the process ΔH and the energy lost to the surroundings TΔSThe difference between the energy produced and the energy lost is the energy available or free to do useful work by the process ΔGIf the process somehow could be made to take place under conditions of thermodynamic. Lesson 9 - Precipitation Reactions.
They are formed when non-metal gains the electrons. RXN1 Describe a chemical reaction using words and symbolic equations. Modification of work by vxlaFlickr.
This is the currently selected item. If youre feeling a bit shaky on all the steps and details you can review the simple method of balancing equationsOtherwise you may wish to review how to balance oxidation-reduction or redox reactions or move on to understanding mole relations. Ionic chemicals involve electrolytes which are substances that dissociate into ions when dissolved in polar solvents.
The method is as follows. Molecular complete ionic and net ionic equations. Here are the steps involved in balancing chemical equations plus a worked example.
Reductionoxidation or redox reactions and acid-base reactions often involve charged species. Figure 11 Chemical substances and processes are essential for our existence providing sustenance keeping us clean and healthy fabricating electronic devices enabling transportation and much more. Predicting Precipitates and Net Ionic Equations Precipitation Reactions.
Take these free online quizzes on maths physics chemistry and enhance your competitive problem-solving skills. They gain one or more than one electron and do not lose any protons. Therefore they possess a net negative charge.
Free chemistry balancing equations teaching resources chemical reactions worksheet ks3 science resource grade 5 writing from word. Modification of work by the Italian voiceFlickr. _____ Writing and Balancing Equations Worksheet STO1 Balance a chemical equation.
JEE Mains Chapter wise Practice Questions Last 30 Years - examsnet. Lesson 9 - Precipitation Reactions. Predict the product of the following reaction with a chemical equation calculator.
There are Two Methods for Balancing a Redox Reaction Namely. How To Write Net Ionic Equations Step by Step. Therefore they possess a net positive charge.
A K 4 FeCN 6 b H 2 SO 4 c H 2 O d K 2 SO 4 e FeSO 4 f NH 4 2 SO 4. STO2 Identify the parts of a chemical equation. This method is mainly being used for balancing redox reactions in acid on the basis of oxidation numbers.
Anions are negatively charged ions. Balancing chemical equations 1 Get 3 of 4 questions to level up. Complete ionic and net ionic equations Opens a modal 2015 AP Chemistry free response 3a Opens a modal Up next for you.
Vedantus online quiz is curated by the best subject matter experts. In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero.

Oxidation Reduction Redox Reactions Balancing Redox Reactions Chemistry Net Chemistry Lessons Teaching Chemistry Chemistry Classroom
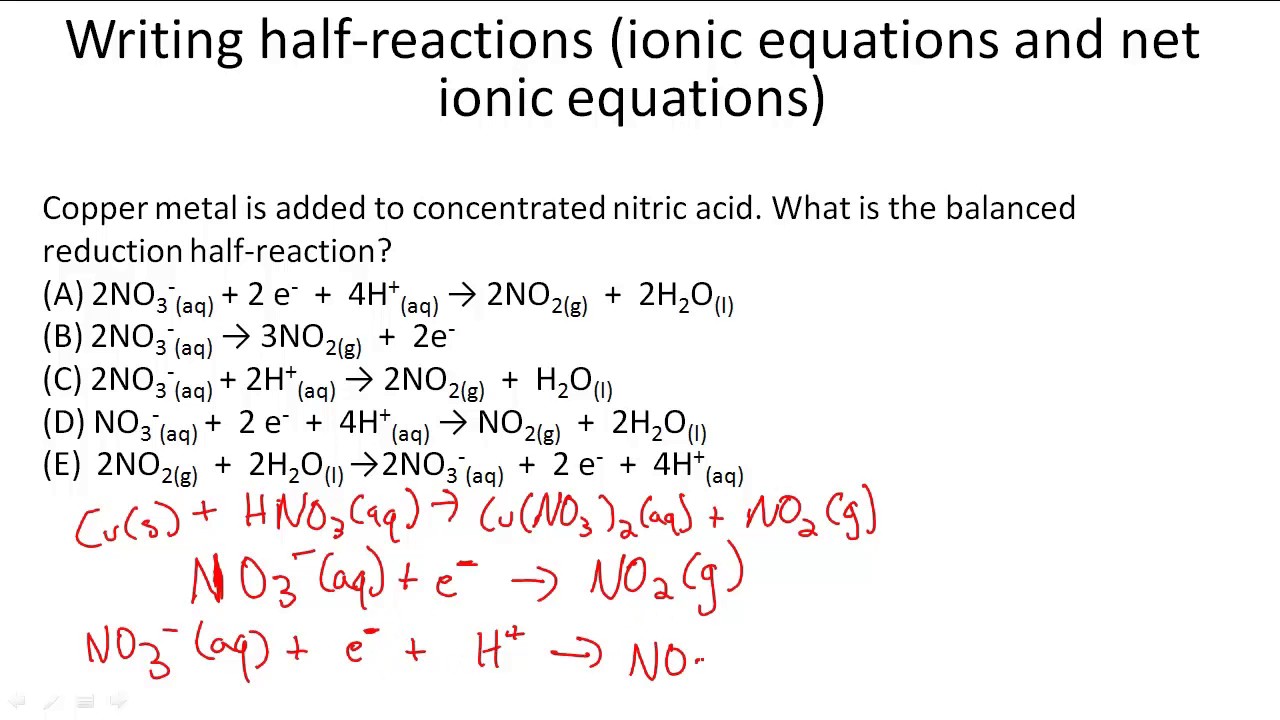
Writing Half Reactions Ionic Equations And Net Ionic Equations Youtube
No comments for "Balancing Net Ionic Redox Equations"
Post a Comment